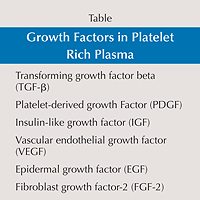
 There are many different proprietary methods of creating platelet-rich plasma but the essential concepts behind creating the platelet-rich plasma are similar for each system. The platelet-rich plasma contains platelets as well as specific growth factors (Table). These growth factors include: transforming growth factor beta (TGF-?), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and fibroblast growth factor-2 (FGF-2). Many of these factors have been shown to enhance one or more phases of bone and soft tissue healing. Insulin-like growth factor is thought to stimulate osteoblast proliferation and differentiation. Platelet-derived growth factor, EGF, and FGF-2 have been shown to stimulate proliferation of osteoblastic progenitors as well as to affect the mitogenesis of mesenchymal stem cells and to stimulate epidermal cell proliferation. Transforming growth factor beta is believed to stimulate collagen synthesis. Angiogenic factors, including VEGF and FGF-2, are believed to enhance early angiogenesis and revascularization.1
There are many different proprietary methods of creating platelet-rich plasma but the essential concepts behind creating the platelet-rich plasma are similar for each system. The platelet-rich plasma contains platelets as well as specific growth factors (Table). These growth factors include: transforming growth factor beta (TGF-?), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and fibroblast growth factor-2 (FGF-2). Many of these factors have been shown to enhance one or more phases of bone and soft tissue healing. Insulin-like growth factor is thought to stimulate osteoblast proliferation and differentiation. Platelet-derived growth factor, EGF, and FGF-2 have been shown to stimulate proliferation of osteoblastic progenitors as well as to affect the mitogenesis of mesenchymal stem cells and to stimulate epidermal cell proliferation. Transforming growth factor beta is believed to stimulate collagen synthesis. Angiogenic factors, including VEGF and FGF-2, are believed to enhance early angiogenesis and revascularization.1Growth factors in PRP and their biological functions
| Name | Abbreviation | Function |
|---|---|---|
| Platelet derived growth factor | PDGF | Enhances collagen synthesis, proliferation of bone cells, fibroblast chemotaxis and proliferative activity, macrophage activation |
| Transforming growth factor ß | TGF-ß | Enhances synthesis of type I collagen, promotes angiogenesis, stimulates chemotaxis of immune cells, inhibits osteoclast formation and bone resorption |
| Vascular endothelial growth factor | VEGF | Stimulates angiogenesis, migration and mitosis of endothelial cells, increases permeability of the vessels, stimulates chemotaxis of macrophages and neutrophils |
| Epidermal growth factor | EGF | Stimulates cellular proliferation, differentiation of epithelial cells, promotes cytokine secretion by mesenchymal and epithelial cells |
| Insulin-like growth factor | IGF | Promotes cell growth, differentiation, recruitment in bone, blood vessel, skin and other tissues, stimulates collagen synthesis together with PDGF |
| Fibroblast growth factor | FGF | Promotes proliferation of mesenchymal cells, chondrocytes and osteoblasts, stimulates the growth and differentiation of chondrocytes and osteoblasts |
What Is Prolotherapy?
In a new study from July 2016, Ross Hauser, MD et al. published findings that summarized the benefits of Prolotherapy.
Prolotherapy is a “new” old treatments that has been utilized in clinical practices for over 80 years. Standardized and reviewed in clinical application by Dr. George Hackett in the 1950s, prolotherapy has been shown to be an effective treatment in patients who suffer from joint instability due to ligament damage and overuse and related musculoskeletal and osteoarthritis.
Prolotherapy’s popularity as a treatment for chronic pain has intensified over the past two decades among both physicians and patients as clinical and anecdotal observations has proved in many cases its reliance as a non-surgical option for joint and back pain.
Prolotherapy is a nonsurgical regenerative injection technique that introduces small amounts of an irritant solution to the site of painful and degenerated tendon insertions (entheses), joints, ligaments, and in adjacent joint spaces during several treatment sessions to promote growth of normal cells and tissues.
Irritant solutions most often contain dextrose (d-glucose), a natural form of glucose normally found in the body, but may also contain combinations of human growth hormone, or ozone.
In severe cases, autologous cellular solutions may also be needed, such as platelet-rich plasma (PRP), bone marrow aspirate.
For the patient, the goal of prolotherapy in chronic musculoskeletal pain and instability is the stimulation of body’s natural healing and regenerative processes in the joint that will facilitate the repair and regrowth of connective tissue, ligaments, tendons for tensile strength, and cartilage and other joint stabilizing structures such as labral tissue.
Prolotherapy works by exactly the same process that the human body naturally uses to stimulate the body’s healing system, a process called inflammation. The technique involves the injection of a proliferant (a mild irritant solution) that causes an inflammatory response which “turns on” the healing process. The growth of new ligament and tendon tissue is then stimulated. The ligaments and tendons produced after prolotherapy appear much the same as normal tissues, except that they are thicker, stronger, and contain fibers of varying thickness, testifying to the new and ongoing creation of tissue. The ligament and tendon tissue which forms as a result of prolotherapy is thicker and stronger than normal tissue, up to 40% stronger in some cases!
When is prolotherapy used?
Prolotherapy can be traced back about a hundred years when doctors used to give irritants to help heal many things. But its modern use has really been going since the 1950’s. It was first called ‘sclerotherapy’ as it was thought to be a scar forming therapy, and has been growing in popularity, for the treatment of acute ligament injuries, where the ligament has undergone a mechanical failure leading to laxity but not rupture, these are commonly seen in sports with the ankle and the knee ligaments being the most commonly injured areas.
Ligament laxity after a injury can be present for a long time afterwards and in some cases can be permanent leading to chronic joint instability and cause long-term pain and loss of function. This often prevents a returning to activity long after the original injury has healed. So having a treatment that could speed up the healing rate, but also reduce ligament and joint laxity seems a very attractive prospect to any medical professionals, especially those working in sport, where the speed of an injured player returning to play is often the main measure of your success.
So what does prolotherapy do?
Prolotherapy acts as a local irritant and so creates an increased inflammatory response, this increases protein synthesis and collagen formation and so increased cell proliferation. Prolotherapy is also thought to increase the infiltration of leukocyte (white blood cells) and macrophages (debris removers) as well as increase platelet-derived growth factor (PDGF) and interleukin-1β (IL-1β) (chemical building blocks) and so help improve a ligaments strength, mass, thickness and a trend toward an increase in cell number, glycosaminoglycan (protien), and water content. (source, source , source)
How does prolotherapy feel?
Well the first thing to mention is that prolotherapy can be mildly painful, more so in some areas than others, we have had adult sports men and women have injections in their lateral ankle ligaments, knee MCL’s and LCL’s, lumbar facet joints, SIJ’s, and symphisis pubis injected. They ALL complained of increased pain and discomfort during, and after these injections.
Thats not really surprising as the irritant nature of the solution causes a local inflammation which obviously can cause pain and discomfort, in my experience this lasts anywhere from 36 hours, up to a week afterwards. Patients are advised to rest and take analgesia as required, but obviously not anti-inflammatory medications as this counter effects the work of the injections, they are also advised not to do any vigorous exercise or have any manual therapy during this reactive inflammatory stage. Once the pain and inflammation is settled the ligament or joint is reassessed for laxity and pain and either treated again or begin a rehab program.
Are there any adverse effects to prolotherapy.
In the scant literature and in our clinical experience there are no significant side effects apart for a post injection flare of pain and some tenderness around the injection site. Rabago et al. 2010 did find some very rare effects such as allergic reactions and nerve irritation but none classed as serious.
Abstract
An inflammatory milieu breaks down the cartilage matrix and induces chondrocyte apoptosis, resulting in cartilage destruction in patients with cartilage degenerative diseases, such as rheumatoid arthritis or osteoarthritis. Because of the limited regenerative ability of chondrocytes, defects in cartilage are irreversible and difficult to repair. Mesenchymal stem cells (MSCs) are expected to be a new tool for cartilage repair because they are present in the cartilage and are able to differentiate into multiple lineages of cells, including chondrocytes. Although clinical trials using MSCs for patients with cartilage defects have already begun, its efficacy and repair mechanisms remain unknown.

Figure 1. Strategies for efficient cartilage repair therapy by intra-articular injection of mesenchymal stem cells (MSCs). The inflammatory environment is present in the joints affected by rheumatoid arthritis (RA) or osteoarthritis (OA). Chondrogenic differentiation of intra-articular injected MSCs is inefficient under inflammatory conditions. Therefore, pre procedural treatment with anti-inflammatory drugs and/or chondrogenic agents should be important for efficient cartilage repair therapy.
The inflammatory milieu is known to cause cartilage destruction by causing disturbances in the anabolic/catabolic balance of the cartilage matrix. The inflammatory milieu has also been understood to exert a suppressive action during the process of differentiation from progenitor MSCs to chondrocytes. Therefore, sufficient suppression of articular inflammation prior to a procedure may be important for efficient regeneration of the cartilage in regenerative therapies using MSCs. While continuous studies to develop more efficient techniques for chondrogenic differentiation using MSC-based cell therapies are important, it is necessary to establish a protocol for the improvement and maintenance of the intra-articular environment, where MSCs are transferred, to be suitable for cartilage regeneration.
In several clinical trials(1), MSCs injected into the joints by the least-invasive intra-articular method, and were expected to differentiate into chondrocytes locally in the joint. Whether or not the intra-articular environment of the joints was suitable for chondrogenic differentiation of MSCs is a major concern. As mentioned , cartilage regenerative therapy is likely to be indicated when the affected joints in patients with OA and RA present with inflammatory reactions. However, the inflammatory milieu, including proinflammatory cytokines, strongly inhibits MSC differentiation into chondrocytes. On the other hand, it has been demonstrated that the influence of inflammation (pro inflammatory cytokines) on MSC is differentiation into osteoblasts, resulting in the formation of calcifications. These findings may indicate the risk of developing osteophytes and ectopic calcifications when MSCs are injected into the joint in an inflammatory milieu. Therefore, sufficient suppression of articular inflammation prior to any procedure is important for efficient cartilage regeneration while using MSCs in cartilage regenerative therapy. This can be achieved by a combination of an existing anti-inflammatory agent or a biologic targeting proinflammatory cytokine and a chondro-enhancing agent (Figure 1).
1. Acquiring Chondrocyte Phenotype from Human Mesenchymal Stem Cells under Inflammatory Conditions
Masahiro Kondo, Kunihiro Yamaoka and Yoshiya Tanaka
Int J Mol Sci. 2014 Nov; 15(11): 21270–21285.